Requirements For Processes and Controls
Below are the processes and controls requirements for FDA regulated food businesses.
General Requirements
1. All operations involving food must align with sanitation principles.
2. Quality control must be used to ensure food and packaging is safe.
3. One or more competent individuals must be responsible for sanitation
4. You must protect your food from allergens and contamination.
5. Testing must be used to identify sanitation failures or possible product contamination.
6. Any contaminated food must be discarded or treated to eliminate the contamination.
Raw Materials & Ingredients
1. Ingredients must be inspected for cleanliness and stored safely. If necessary, raw materials should be washed using clean water.
2. Ingredients must be safe for consumption or treated to make them safe (i.e. washed or cooked).
3. Ingredients susceptible to toxins must comply with FDA regulations.
4. Ingredients that are contaminated must comply with FDA regulations if they are to be used.
5. Ingredients must be held in containers that prevent contamination and at an acceptable temperature and humidity level.
6. Frozen ingredients must be kept frozen.
7. Ingredients stored in bulk must be safe from contamination.
8. Ingredients that contain allergens must be identified and held in a way that prevents cross-contact.
Manufacturing Operations:
1. Equipment must be maintained in clean condition.
2. All operations should be controlled to minimize growth of bacteria, contamination and spoilage.
3. Food requiring refrigeration must be refrigerated throughout the operation.
4. Measures used to prevent bacteria growth (i.e. cooking, sterilizing, refrigerating) must be adequate.
5. Re-work must prevent contamination and bacteria growth.
6. When ingredients are unprotected they must not be handled in a way that could cause contamination. Food on conveyor belts must be protected.
7. Equipment, containers, and utensils must be constructed and used in a way that doesn’t contaminate food.
8. You must take measures to protect your product from metal or foreign objects.
9. Contaminated food must:
i. Be disposed of OR
ii. Re-worked and re-examined,
10. Food that is being processed must be protected from contamination.
11. Heat blanching --if used-- must be performed properly
12. Foods that are used repeatedly (i.e. dipping sauces, breading) must be protected from contamination and bacteria growth
13. Filling, assembling, and packaging processes must not contaminate food.
14. Dry foods that rely on low moisture for safety must be sufficiently dry.
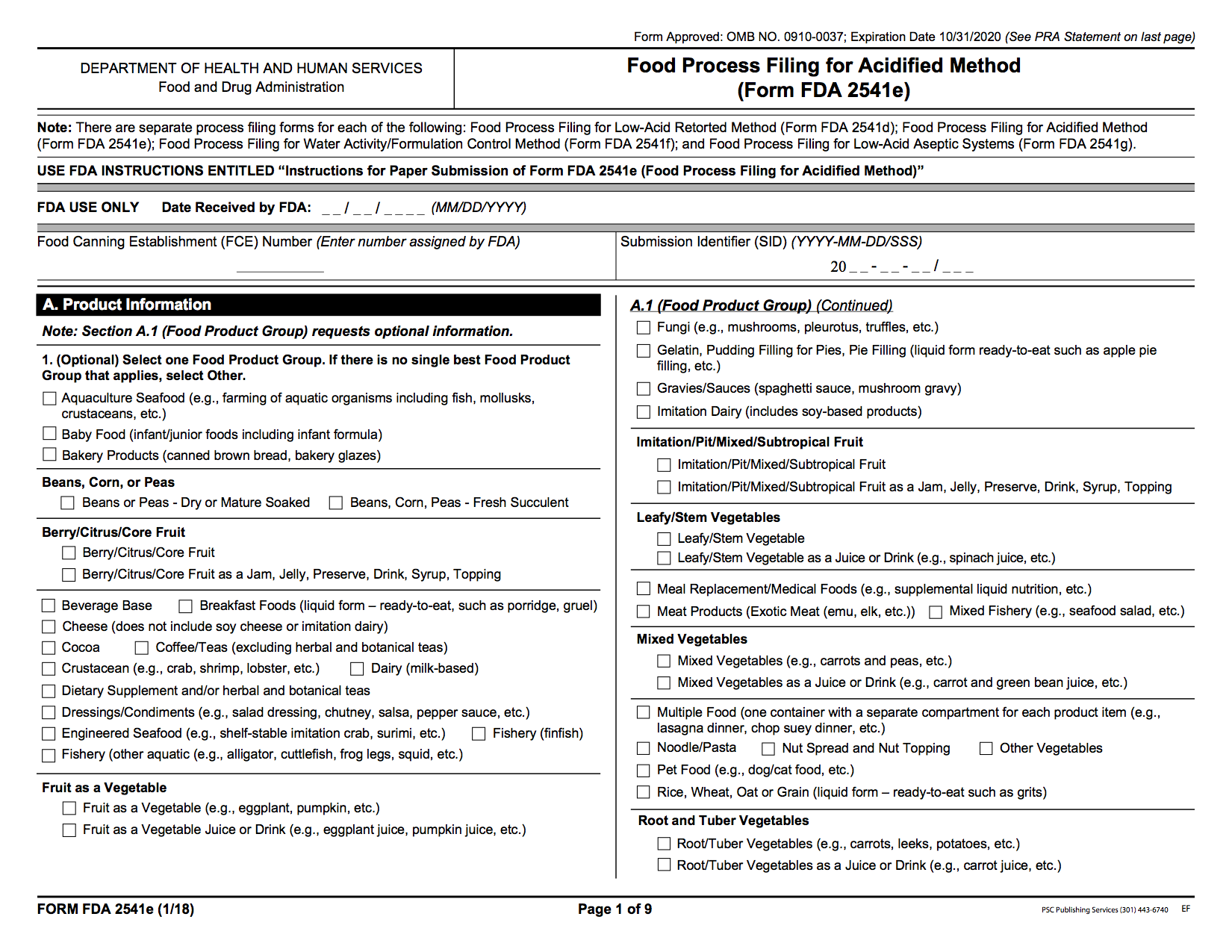
15. Acidified foods that rely on acid for safety must be sufficiently acidic (pH≤4.6)
16. Ice that touches food must be food quality.