What You Need to Know
There are 4 major types of product claims. Each one has specific requirements.
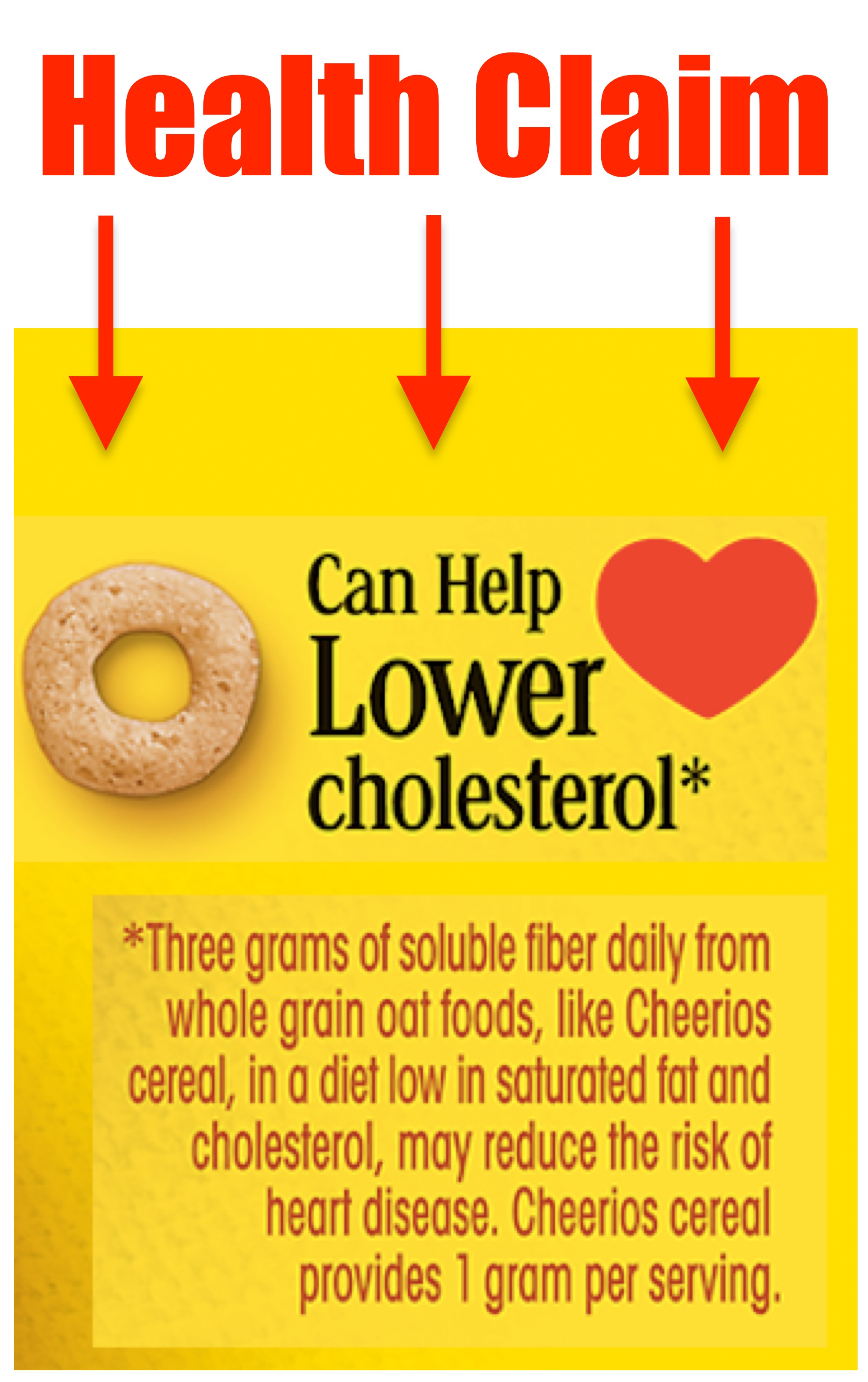
Health Claim: Describes a relationship between a food and a reduced risk of a disease or a health-related condition.
Authorized Health Claims:A health claim that the FDA has authorized for use based on "significant scientific agreement" on the subject.
Qualified Health Claims: A health claim is one that is supported by scientific evidence but does not achieve the "significant scientific agreement" standard. The FDA authorizes Qualified Health Claims for use only when specific wording is applied.
Nutrient Content Claims describe the level of a nutrient in a food.
Structure/Function Claims: describe the role of a nutrient or ingredient on the structure or function of the human body.
Health Claims
A health claim describes a relationship between a food and a reduced risk of a disease or a health-related condition. This can be made in words, images (i.e. a heart), or a reference to a 3rd party certification.
Example of a Health Claim: "Diets low in sodium may reduce the risk of high blood pressure, a disease associated with many factors."
Not a Health Claim: "fruits and vegetables contribute to good dietary health" This is not a health claim because no assertion is made about the reduced risk of a disease or health condition. Instead, this statement would simply be called 'dietary guidance'.)
Types of Health Claims
There are two types of health claims that appear on food labels and marketing. They are:
Requirements for a Health Claim
Health claims cannot be made about the diagnosis, cure, mitigation or treatment of diseases (this is a drug claim)
They must be complete, truthful and not misleading.
Certain foods may be disqualified from health claims based on nutrient levels that are deemed unhealthy. See §101.14 (4)
Health claims must be associated with a risk or health related condition for which the US population or a subgroup (i.e. the elderly) is at risk.
The substance that is the subject of the health claim must have a taste, aroma or nutrient value when consumed at the levels used to justify the claim.
Authorized Health Claims
See main article Authorized Health Claims
What is an Authorized Health Claim?
An Authorized Health Claim describes a health claim that has been reviewed by the FDA and approved for use.
There must be a strong scientific case in order for the FDA to issue an Authorized Health Claim.
When there is emerging evidence between a food and a reduced risk of disease or health condition the FDA will allow the use of a "Qualified Health Claim" in food labeling.
What Health Claims Can I Use On My Product Label?
See full article Authorized Health Claims You Can Use On Your Label
Example of a Qualified Health Claim
You may use an Authorized Health Claim or Qualified Health Claim that has already been approved by the FDA. For examples, see the 2013 FDA Labeling Guide. The wording requirements for Authorized Health Claims offer some flexibility in how that claim is stated.
Additionally, anyone may submit a notification to the FDA of a health claim based on scientific studies conducted by certain branches of the US government. This is called a Health Claim Based on an Authoritative Statement by a Scientific Body.
The FDA may approve the health claim, reject it, or issue a Qualified Health Claim with specific guidelines about how the claim can be used.
Qualified Health Claims
What is a Qualified Health Claim?
A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements.
When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
Nutrient Content Claims
Nutrient Content Claims
Main Article: Nutrient Content Claims
Examples of Nutrient Content Claims
What is a Nutrient Content Claim?
A Nutrient Content Claim is a claim that characterizes the level of a nutrient in the food.
This is different than information listed in the Nutrition Information Panel. Any claims made about nutrition outside of the Nutrition Information Panel would be considered Nutrient Content Claims.
Structure/Function Claims
What is a Structure/Function Claim?
A Structure/Function Claim describe the role of a nutrient or ingredient on the structure or function of the human body. These may appear on the labels of foods, dietary supplements or drugs.
Examples of a Structure/Function Claim:
"Calcium builds strong bones"
"Fiber maintains bowel regularity"
"Antioxidants maintain cell integrity"
Conventional food producers do not need to notify the FDA about structure/function claims or make disclaimers associated with these claims on their product labels. Producers of Dietary Supplements may face additional requirements.
Categories of Structure/Function Claims:
These claims are only associated with producers of Dietary Supplements.
Claims of General Well-Being: These describe general well-being from consumption of a nutrient or ingredient.
Nutrient-Deficiency Disease Claims: These describe a benefit related to a nutrient deficiency disease (such as vitamin C and scurvey).
These claims are only allowed if the claim also states how prevalent the disease is in the US.
A business making a nutrient-deficiency disease claim must submit a notification that includes the text of the claim to the FDA no later than 30 days after marketing the dietary supplement that contains the claim.