The Basics: pH Meters
pH meters are a common tool in the food processing context.
Since acidity is often used as a means to preserve foods, a pH meter is required to confirm that the correct level of acidity is achieved in the product.
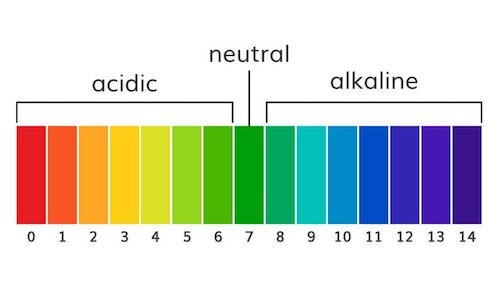
A pH meter measures the acidity of a substance on a scale from acidic to alkaline (a.k.a. basic).
pH is typically measured on a numeric scale from 1 -14 but it can also be measured using a color gradient.
Whether you’re taking pH readings so you can test a chemical concentration or as part of your HACCP plan.
pH range is measured from 0 (acidic) to 14 (alkaline)
Do I need a pH Meter?
You need a pH meter if the following circumstances apply:
You produce one of the following types of food:
Acidified food
Fermented food
Low-acid-canned-food
You use chemicals whose concentrations require testing using a pH meter.
You produce a product that uses acid or fermentation as a means of preservation.
Considerations When Choosing a pH Tester
Accuracy: Do you need accuracy to 0.1 or 0.02? This is probably the biggest factor in what you should buy.
Calibration: Is it easy to quickly re-calibrate (you’ll probably have to calibrate your device before each use if you are recording your results for a HACCP/Food Safety Plan).
Portability: Does your pH meter need to be portable or can you choose a plug-in bench top model? Note that smaller, more portable pH testers tend to be less accurate.
Handheld pH Meter
What’s it for: Taking pH measurements when you need accurate results and portability. Perfect for the food processor who needs to take pH readings for a HACCP / food safety plan.
Pros:
Higher degree of accuracy (typically 0.01-0.03) than pen-style models. Accurate enough for all food processing applications.
Portable
Replaceable electrode.
Cons:
Two-hand operations is slightly less convenient than pen-style.
Why We Picked Our Winner:
High degree of accuracy (Accurate ±0.01)
Automatic temperature compensation
We’ve been using this model for 3 years with continued accurate results.
Extremely long battery life from a single 9V battery
Durable construction. Trust us, we’ve dropped it.
Pen-Style pH Meter
What’s it for: Taking multiple readings across different areas in a food production space with reasonable accuracy (0.1±). Pen style pH testers are a good place to start if you need reasonable accuracy and are on a budget.
If you need a pH meter as part of your HACCP or Food Safety Plan, consider a more accurate tester see our Handheld and Benchtop choices below.
Pros:
Remarkable portability. Slip it into your pocket.
Reasonably good accuracy for an entry-level price point.
Cons:
Accuracy of ± 0.1 isn’t sufficient for many food processors.
There are lots of cheap pen-style pH testers on the market which are prone to inaccurate results. Consider our pick for Pen Style pH tester
You’ll want to keep buffering solutions with your pH meter so that you can calibrate it. So even if you have a very tiny pH meter, you’ll need to haul around these accessories with you if you want accurate readings.
Why We Picked Our Winner:
Extremely portable
Fully waterproof (this helps in a food processing context).
Best tested accuracy and reliability among the pen-style models we tested. Accurate to ± 0.1
Affordable price point ~($50)
Basic: pH Test Strips
Overview: Test strips are best for simple measurements that do not require a high degree of specificity. You might use a test strip to get a general sense of pH or if you’re only doing occasional tests and will be happy with a general result.
Pros:
Easy use — just dip and compare the strip with the color key on the packaging.
No calibration required
Small size for on-the-go testing
Cons:
Not as accurate as electric pH meters
Reactive paper shows the pH of whatever it contacts — whether that’s what you' want to test or not.
Typically has a maximum 2 year shelf life. After this time accuracy may vary.
Our Choice:
We prefer Hydrion because they’re the most reliable test strip on the market. They’re a little pricier, but you won’t risk buying an expired test strips like some other producers offer. It’s the most accurate test strip on the market. If you want accurate readings then stick with reputable brands.